Chapter: Chemical Bonding and Molecular Structure
0 of 92 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
- 76
- 77
- 78
- 79
- 80
- 81
- 82
- 83
- 84
- 85
- 86
- 87
- 88
- 89
- 90
- 91
- 92
Information
Subject: Chemistry
Syllabus: Chemical Bonding and Molecular Structure
Duration: 1 Hr.
Read the following instruction carefully.
- There are 60 total questions in this test
- Each question has 4 options out of which only one is correct.
- You will be awarded 4 points for each correct answer and 1 point will be deducted for each wrong answer.
- Try not to guess the answer as there is negative marking.
- Your Score & Rank will be shown after submitting the test.
- In this quiz you are going to check your conceptual knowledge.
You have already completed the Test before. Hence you can not start it again.
Test is loading...
You must sign in or sign up to start the Test.
You have to finish following quiz, to start this Test:
Your results are here!! for" JPY_11_Chem_04_04_Chemical Bonding and Molecular Structure "
0 of 92 questions answered correctly
Your time:
Time has elapsed
Your Final Score is : 0
You have attempted : 0
Number of Correct Questions : 0 and scored 0
Number of Incorrect Questions : 0 and Negative marks 0
| Average score |
|
| Your score |
|
-
Level 1
You have attempted: 0
Number of Correct Questions: 0 and scored 0
Number of Incorrect Questions: 0 and Negative marks 0
-
Dear $form{0} you have completed this test.
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
- 76
- 77
- 78
- 79
- 80
- 81
- 82
- 83
- 84
- 85
- 86
- 87
- 88
- 89
- 90
- 91
- 92
- Answered
- Review
-
Question 1 of 92
1. Question
4 pointsWhich of the following compounds contain(s) no covalent bond(s) \(\begin{align}KCl,P{{H}_{3}},{{O}_{2}},{{B}_{2}}{{H}_{6}},{{H}_{2}}S{{O}_{4}}\end{align}\)?
-
Question 2 of 92
2. Question
4 pointsAmongst LiCl, RbCl, \(\begin{align}BeC{{l}_{2}}\text{ }and\text{ }MgC{{l}_{2}}\end{align}\) the compounds with the greatest and the least ionic character, respectively are:
-
Question 3 of 92
3. Question
4 pointsWhich of these statements is not true?
-
Question 4 of 92
4. Question
4 pointsLattice energy of an ionic compound depends upon
-
Question 5 of 92
5. Question
4 pointsortho-Nitrophenol is less soluble in water than p- and m- nitrophenols because:
-
Question 6 of 92
6. Question
4 pointsAmong the following, the species having the smallest bond length is
-
Question 7 of 92
7. Question
4 pointsWhich one of the following pairs of species have the same bond order?
-
Question 8 of 92
8. Question
4 pointsWhich of the following hydrogen bonds is strongest?
-
Question 9 of 92
9. Question
4 pointsThe bond order in NO is 2.5 while that in \(\begin{align}N{{O}^{+}}\end{align}\) is 3. Which of the following statements is true for these two species?
-
Question 10 of 92
10. Question
4 pointsAn ether is more volatile than an alcohol having the same molecular formula. This is due to
-
Question 11 of 92
11. Question
4 pointsThe dipole moments of \(\begin{align}CC{{l}_{4}},\text{ }CHC{{l}_{{3\text{ }}}}and\text{ }C{{H}_{4}}\end{align}\) are in the order:
-
Question 12 of 92
12. Question
4 pointsWhich compound exhibits maximum dipole moment among the following?
-
Question 13 of 92
13. Question
4 pointsMolecule AB has a bond length of 1.617Å and a dipole moment of 0.38 D. The fractional charge on each atom (absolute magnitude) is: (\(\displaystyle {{e}_{0}}=4.802\times {{10}^{{-10}}}esu\))
-
Question 14 of 92
14. Question
4 pointsThe correct order of bond dissociation energy among \(\begin{align}{{N}_{2}},~{{O}_{2}},\text{ }O_{2}^{-}\end{align}\) is shown in which of the following arrangements?
-
Question 15 of 92
15. Question
4 pointsWhich one of the following molecules is polar?
-
Question 16 of 92
16. Question
4 pointsBond distance in HF is m. Dipole moment of HF is Cm. The percentage ionic character in HF will be: (electron charge = C)
-
Question 17 of 92
17. Question
4 pointsAmong the following chloro-compound having the lowest dipole moment is
-
Question 18 of 92
18. Question
4 pointsAlthough ion and \(\begin{align}{{N}_{2}}\end{align}\) molecule are isoelectronic, yet \(\begin{align}{{N}_{2}}\end{align}\) molecule is chemically inert because of
-
Question 19 of 92
19. Question
4 pointsThe charge/size ratio of a cation determines its polarizing power. Which one of the following sequences represents the increasing order of the polarizing power of the cationic species, \(\begin{align}{{K}^{+}},C{{a}^{{2+}}},M{{g}^{{2+}}},B{{e}^{{2+}}}\end{align}\)?
-
Question 20 of 92
20. Question
4 pointsWhich one of the following pairs of molecules will have permanent dipole moments for both members?
-
Question 21 of 92
21. Question
4 pointsThe compound that has the largest H – M – H bond angle (M = N, S, C), is:
-
Question 22 of 92
22. Question
4 pointsThe molecule in which hybrid MOs involve only one d-orbital of the central atom is:
-
Question 23 of 92
23. Question
4 pointsIf \(\begin{align}A{{B}_{4}}\end{align}\) molecule is a polar molecule, a possible geometry of \(\begin{align}A{{B}_{4}}\end{align}\) is:
-
Question 24 of 92
24. Question
4 pointsThe shape/ structure of and \(\begin{align}Xe{{O}_{3}}{{F}_{2}}\end{align}\), respectively, are:
-
Question 25 of 92
25. Question
4 pointsThe molecular geometry of \(\begin{align}S{{F}_{6}}\end{align}\) is octahedral. What is the geometry of \(\begin{align}S{{F}_{4}}\end{align}\) (including lone pair(s) of electrons, if any)?
-
Question 26 of 92
26. Question
4 pointsThe correct statement about \(\begin{align}IC{{l}_{5}}\end{align}\) and \(\begin{align}ICl_{4}^{-}\end{align}\) is:
-
Question 27 of 92
27. Question
4 pointsThe ion that has \(\begin{align}s{{p}^{3}}{{d}^{2}}\end{align}\) hydridisation for the central atom, is:
-
Question 28 of 92
28. Question
4 pointsTotal number of lone pair of electrons in \(\begin{align}I_{3}^{-}\end{align}\) ion is :
-
Question 29 of 92
29. Question
4 pointsWhich of the following conversions involves change in both shape and hybridisation?
-
Question 30 of 92
30. Question
4 pointsThe incorrect geometry is represented by __________.
-
Question 31 of 92
31. Question
4 pointsIdentify the pair in which the geometry of the species is Tshape and square pyramidal, respectively
-
Question 32 of 92
32. Question
4 pointsThe decreasing order of bond angles in \(\begin{align}B{{F}_{3}},\text{ }N{{H}_{3}},\text{ }P{{F}_{3}}\text{ }and~I~_{3}^{-}\end{align}\) is:
-
Question 33 of 92
33. Question
4 points\(\begin{align}s{{p}^{3}}{{d}^{2}}\end{align}\) Hybridisation is not displayed by:
-
Question 34 of 92
34. Question
4 pointsThe group having triangular planar structures is:
-
Question 35 of 92
35. Question
4 pointsThe species in which the N atom is in a state of sp hybridisation is:
-
Question 36 of 92
36. Question
4 pointsThe group of molecules having identical shape is:
-
Question 37 of 92
37. Question
4 pointsThe bond angle H–X–H is the greatest in the compound:
-
Question 38 of 92
38. Question
4 pointsThe number and type of bonds in \(\begin{align}C_{2}^{{2-}}\end{align}\) ion in \(\begin{align}Ca{{C}_{2}}\end{align}\) are:
-
Question 39 of 92
39. Question
4 pointsWhich of the following molecules has two sigma (\(\begin{align}\sigma \end{align}\)) and two pi (\(\begin{align}\pi \end{align}\)) bonds?
-
Question 40 of 92
40. Question
4 pointsThe shape of \(\begin{align}IF_{6}^{-}\end{align}\) is:
-
Question 41 of 92
41. Question
4 pointsIn which of the following sets, all the given species are isostructural?
-
Question 42 of 92
42. Question
4 pointsIn which of the following pairs, the two species are not isostructural?
-
Question 43 of 92
43. Question
4 pointsThe formation of molecular complex results in a change in hybridisation of boron
-
Question 44 of 92
44. Question
4 pointsWhich of the following has the square planar structure?
-
Question 45 of 92
45. Question
4 pointsAmong the following species which two have trigonal bipyramidal shape?
(I) \(\begin{align}N{{I}_{3}}\end{align}\)
(II) \(\begin{align}I_{3}^{-}\end{align}\)
(III) \(\begin{align}SO_{3}^{{2-}}\end{align}\)
(IV) \(\begin{align}NO_{3}^{-}\end{align}\) -
Question 46 of 92
46. Question
4 pointsThe bond dissociation energy of B – F in \(\begin{align}B{{F}_{3}}\end{align}\) is 646 kJ \(\begin{align}mo{{l}^{{-1}}}\end{align}\), whereas that of C – F in \(\begin{align}C{{F}_{4}}\end{align}\) is 515 kJ \(\begin{align}mo{{l}^{{-1}}}\end{align}\). The correct reason for higher B – F bond dissociation energy as compared to that of C – F bond is
-
Question 47 of 92
47. Question
4 pointsIn which of the following molecules/ions are all the bonds not equal?
-
Question 48 of 92
48. Question
4 pointsThe decreasing values of bond angles from \(\begin{align}N{{H}_{{3\text{ }}}}\left( {106{}^\text{o}} \right)\text{ }to~Sb{{H}_{{3\text{ }}}}\left( {101{}^\text{o}} \right)\end{align}\) down group-15 of the periodic table is due to
-
Question 49 of 92
49. Question
4 pointsThe correct order of bond angles (smallest first) in \(\begin{align}{{H}_{2}}S,~N{{H}_{3}},\text{ }B{{F}_{3}}\text{ }and\text{ }Si{{H}_{4}}\end{align}\) is
-
Question 50 of 92
50. Question
4 pointsThe states of hybridization of boron and oxygen atoms in boric acid (\(\begin{align}{{H}_{3}}B{{O}_{3}}\end{align}\)) are respectively
-
Question 51 of 92
51. Question
4 pointsWhich one of the following has the regular tetrahedral structure?
-
Question 52 of 92
52. Question
4 pointsThe maximum number of 90º angles between bond pairbond pair of electrons is observed in
-
Question 53 of 92
53. Question
4 pointsWhich one of the following compounds has the smallest bond angle in its molecule?
-
Question 54 of 92
54. Question
4 pointsThe pair of species having identical shapes for molecules of both species is
-
Question 55 of 92
55. Question
4 pointsIn which of the following species the interatomic bond angle is 109° 28’?
-
Question 56 of 92
56. Question
4 pointsHybridisation of the underline atom changes in:
-
Question 57 of 92
57. Question
4 pointsThe potential energy curve for the H2 molecule as a function of internuclear distance is:
-
Question 58 of 92
58. Question
4 pointsThe structure of PCl5 in the solid state is:
-
Question 59 of 92
59. Question
4 pointsOf the species, the one with minimum bond strength is:
-
Question 60 of 92
60. Question
4 pointsIf the magnetic moment of a dioxygen species is 1.73 B.M, it may be:
-
Question 61 of 92
61. Question
4 pointsThe bond order and the magnetic characteristics of are:
-
Question 62 of 92
62. Question
4 pointsDuring the change of \(\begin{align}{{O}_{2}}\text{ to }O_{2}^{-}\end{align}\), the incoming electron goes to the orbital:
-
Question 63 of 92
63. Question
4 pointsAmong the following, the molecule expected to be stabilised by anion formation is: \(\begin{align}{{C}_{2}},\text{ }{{O}_{2}},\text{ }NO,\text{ }{{F}_{2}}~\end{align}\)
-
Question 64 of 92
64. Question
4 pointsAmong the following species, the diamagnetic molecule is:
-
Question 65 of 92
65. Question
4 pointsAmong the following molecules/ions,
\(\begin{align}C_{2}^{{2-}},N_{2}^{{2-}},O_{2}^{{2-}},{{O}_{2}}\end{align}\)
Which one is diamagnetic and has the shortest bond length? -
Question 66 of 92
66. Question
4 pointsTwo pi and half sigma bonds are present in:
-
Question 67 of 92
67. Question
4 pointsAccording to molecular orbital theory, which of the following is true with respect to \(\begin{align}L{{i}_{2}}^{+}\end{align}\) and \(\begin{align}L{{i}_{2}}^{-}\end{align}\)?
-
Question 68 of 92
68. Question
4 pointsIn which of the following processes, the bond order has increased and paramagnetic character has changed to diamagnetic?
-
Question 69 of 92
69. Question
4 pointsAccording to molecular orbital theory, which of the following will not be a viable molecule?
-
Question 70 of 92
70. Question
4 points\(\begin{align}\begin{array}{l}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,(I)\,\,\,\,(II)\\H-N—N—N~\end{array}\end{align}\)
In hydrogen azide, the bond orders of bonds (I) and (II) are _________. -
Question 71 of 92
71. Question
4 pointsWhich of the following best describes the diagram of molecular orbital?
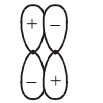
-
Question 72 of 92
72. Question
4 pointsIn the molecular orbital diagram for the molecular ion, \(\begin{align}N_{2}^{+}\end{align}\), the number of electrons in the \(\begin{align}{{\sigma }_{{2p}}}\end{align}\) molecular orbital is:
-
Question 73 of 92
73. Question
4 pointsWhich of the following is paramagnetic?
-
Question 74 of 92
74. Question
4 pointsWhich of the following species is not paramagnetic?
-
Question 75 of 92
75. Question
4 pointsAfter understanding the assertion and reason, choose the correct option.
Assertion: In the bonding molecular orbital (MO) of H2, electron density is increased between the nuclei.
Reason: The bonding MO is \(\begin{align}{{\psi }_{A}}+{{\psi }_{B}}\end{align}\), which shows destructive interference of the combining electron waves. -
Question 76 of 92
76. Question
4 pointsWhich one of the following properties is not shown by NO?
-
Question 77 of 92
77. Question
4 pointsWhich one of the following molecules is paramagnetic?
-
Question 78 of 92
78. Question
4 pointsWhich of the following has unpaired electron(s)?
-
Question 79 of 92
79. Question
4 pointsIn which of the following pairs of molecules/ions, both the species are not likely to exist?
-
Question 80 of 92
80. Question
4 pointsWhich one of the following molecules is expected to exhibit diamagnetic behaviour?
-
Question 81 of 92
81. Question
4 pointsWhich of the following is the wrong statement
-
Question 82 of 92
82. Question
4 pointsStability of the species \(\begin{align}L{{i}_{2}},\text{ }Li_{2}^{-}\text{ }and\text{ }Li_{2}^{+}\end{align}\) increases in the order of:
-
Question 83 of 92
83. Question
4 pointsIn which of the following ionization processes the bond energy has increased and also the magnetic behaviour has changed from paramagnetic to diamagnetic?
-
Question 84 of 92
84. Question
4 pointsBond order normally gives idea of stability of a molecular species. All the molecules viz. \(\begin{align}{{H}_{2}},\text{ }L{{i}_{2}}\text{ }and\text{ }{{B}_{2}}\end{align}\)have the same bond order yet they are not equally stable. Their stability order is
-
Question 85 of 92
85. Question
4 pointsThe internuclear distances in O – O bonds for \(\begin{align}O_{2}^{+},{{O}_{2}},O_{2}^{-}\end{align}\) and \(\begin{align}O_{2}^{{2-}}\end{align}\) respectively are:
-
Question 86 of 92
86. Question
4 pointsThe number of types of bonds between two carbon atoms in calcium carbide is:
-
Question 87 of 92
87. Question
4 pointsUsing MO theory, predict which of the following species has the shortest bond length?
-
Question 88 of 92
88. Question
4 pointsWhich of the following species exhibits the diamagnetic behaviour?
-
Question 89 of 92
89. Question
4 pointsIn which of the following ionization processes, the bond order has increased and the magnetic behaviour has changed?
-
Question 90 of 92
90. Question
4 pointsWhich of the following molecules/ions does not contain unpaired electrons?
-
Question 91 of 92
91. Question
4 pointsWhich of the following species is diamagnetic in nature?
-
Question 92 of 92
92. Question
4 pointsWhich of the following are arranged in an increasing order of their bond strength?
