Chapter: State of Matter
0 of 48 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
Information
Subject: Chemistry
Syllabus: State of Matter
Duration: 1 Hr.
Read the following instruction carefully.
- There are 60 total questions in this test
- Each question has 4 options out of which only one is correct.
- You will be awarded 4 points for each correct answer and 1 point will be deducted for each wrong answer.
- Try not to guess the answer as there is negative marking.
- Your Score & Rank will be shown after submitting the test.
- In this quiz you are going to check your conceptual knowledge.
You have already completed the Test before. Hence you can not start it again.
Test is loading...
You must sign in or sign up to start the Test.
You have to finish following quiz, to start this Test:
Your results are here!! for" JPY_11_Chem_05_05_State of Matter "
0 of 48 questions answered correctly
Your time:
Time has elapsed
Your Final Score is : 0
You have attempted : 0
Number of Correct Questions : 0 and scored 0
Number of Incorrect Questions : 0 and Negative marks 0
| Average score |
|
| Your score |
|
-
Not categorized
You have attempted: 0
Number of Correct Questions: 0 and scored 0
Number of Incorrect Questions: 0 and Negative marks 0
-
Level 1
You have attempted: 0
Number of Correct Questions: 0 and scored 0
Number of Incorrect Questions: 0 and Negative marks 0
-
Dear $form{0} you have completed this test.
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- Answered
- Review
-
Question 1 of 48
1. Question
4 pointsA mixture of one mole each of \(\begin{align}{{H}_{2}}\end{align}\), He and \(\begin{align}{{O}_{2}}\end{align}\) each are enclosed in a cylinder of volume] at temperature. If the partial pressure of \(\begin{align}{{H}_{2}}\end{align}\) is 2 atm, the total pressure of the gases in the cylinder is:
-
Question 2 of 48
2. Question
4 pointsA spherical balloon of radius 3 cm containing helium gas has a pressure of\(\begin{align}48\times {{10}^{{-3}}}\end{align}\)bar. At the same temperature, the pressure, of a spherical balloon of radius 12 cm containing the same amount of gas will be ______ \(\begin{align}\times {{10}^{6}}\end{align}\) bar.
-
Question 3 of 48
3. Question
4 pointsWhich one of the following graphs is not correct for ideal gas?
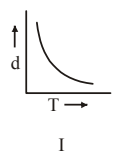
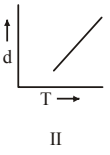
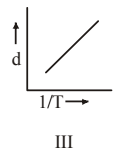
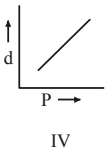
d = Density, P = Pressure, T = Temperature -
Question 4 of 48
4. Question
4 pointsMatch the type of interaction in column A with the distance dependence of their interaction energy in column B:
A B
(I) ion-ion (A) \(\begin{align}\frac{1}{r}\end{align}\)
(II) dipole-dipole (B) \(\begin{align}\frac{1}{{{{r}^{2}}}}\end{align}\)
(III) London dispersion (C) \(\begin{align}\frac{1}{{{{r}^{3}}}}\end{align}\)
. (D) \(\begin{align}\frac{1}{{{{r}^{6}}}}\end{align}\) -
Question 5 of 48
5. Question
4 pointsThe predominant intermolecular forces present in ethyl acetate, a liquid, are:
-
Question 6 of 48
6. Question
4 pointsThe relative strength of interionic/ intermolecular forces in decreasing order is:
-
Question 7 of 48
7. Question
4 points0.5 moles of gas A and x moles of gas B exert a pressure of 200 Pa in a container of volume 10 \(\begin{align}{{m}^{3}}\end{align}\) at 1000 K. Given R is the gas constant in J \(\begin{align}{{K}^{{-1}}}mo{{l}^{{-1}}}\end{align}\), x is:
-
Question 8 of 48
8. Question
4 pointsAn open vessel at 27°C is heated until two fifth of the air (assumed as an ideal gas) in it has escaped from the vessel. Assuming that the volume of the vessel remains constant, the temperature at which the vessel has been heated is:
-
Question 9 of 48
9. Question
4 pointsAssuming ideal gas behaviour, the ratio of density of ammonia to that of hydrogen chloride at same temperature and pressure is: (Atomic wt. of Cl = 35.5u)
-
Question 10 of 48
10. Question
4 points10. Among the following, the incorrect statement is:
-
Question 11 of 48
11. Question
4 pointsAt 300 K, the density of a certain gaseous molecule at 2 bar is double to that of dinitrogen (\(\begin{align}{{N}_{2}}\end{align}\)) at 4 bar. The molar mass of gaseous molecule is:
-
Question 12 of 48
12. Question
4 pointsTwo closed bulbs of equal volume (\(\begin{align}V\end{align}\)) containing an ideal gas initially at pressure \(\begin{align}{{p}_{i}}\end{align}\) and temperature \(\begin{align}{{T}_{1}}\end{align}\) are connected through a narrow tube of negligible volume. The temperature of one of the bulbs is then raised to \(\begin{align}{{T}_{2}}\end{align}\). The final pressure \(\begin{align}{{p}_{f}}\end{align}\) is:
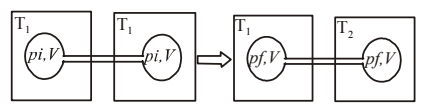
-
Question 13 of 48
13. Question
4 pointsWhen does a gas deviate the most from its ideal behaviour?
-
Question 14 of 48
14. Question
4 pointsThe initial volume of a gas cylinder is 750.0 mL. If the pressure of gas inside the cylinder changes from 840.0 mm Hg to 360.0 mm Hg, the final volume the gas will be:
-
Question 15 of 48
15. Question
4 pointsSulphur dioxide and oxygen were allowed to diffuse through a porous partition. 20 \(\begin{align}d{{m}^{3}}\end{align}\) of \(\begin{align}S{{O}_{2}}\end{align}\) diffuses through the porous partition in 60 seconds. The volume of \(\begin{align}{{O}_{2}}\end{align}\) in \(\begin{align}d{{m}^{3}}\end{align}\) which diffuses under the similar condition in 30 seconds will be (atomic mass of sulphur = 32 u):
-
Question 16 of 48
16. Question
4 pointsFor 1 mol of an ideal gas at a constant temperature \(\begin{align}T\end{align}\), the plot of (log \(\begin{align}P\end{align}\)) against (log \(\begin{align}V\end{align}\)) is a (\(\begin{align}P\end{align}\): Pressure, \(\begin{align}V\end{align}\): Volume)
-
Question 17 of 48
17. Question
4 pointsWhen \(\begin{align}C{{O}_{2}}\end{align}\) (g) is passed over red hot coke it partially gets reduced to CO(g). Upon passing 0.5 L of \(\begin{align}C{{O}_{2}}\end{align}\) (g) over red hot coke, the total volume of the gases increased to 700 mL. The composition of the gaseous mixture at STP is
-
Question 18 of 48
18. Question
4 pointsAn open vessel at 300 K is heated till 2/5th of the air in it is expelled. Assuming that the volume of the vessel remains constant, the temperature to which the vessel is heated, is
-
Question 19 of 48
19. Question
4 pointsThe intermolecular interaction that is dependent on the inverse cube of distance between the molecules is:
-
Question 20 of 48
20. Question
4 pointsIf of water is introduced into a 1.0 \(\begin{align}\text{d}{{\text{m}}^{3}}\end{align}\) flask at 300 K, how many moles of water are in the vapour phase when equilibrium is established? (Given: Vapour pressure of H2O at 300 K is 3170 Pa; Y = 8.314 J\(\begin{align}{{\text{K}}^{{-1}}}\text{ }mol{{~}^{{-1}}}\end{align}\))
-
Question 21 of 48
21. Question
4 pointsFor an ideal gas, number of moles per litre in terms of its pressure \(\begin{align}P\end{align}\), gas constant \(\begin{align}R\end{align}\) and temperature \(\begin{align}T\end{align}\) is
-
Question 22 of 48
22. Question
4 pointsValue of gas constant\(\begin{align}R\end{align}\) is
-
Question 23 of 48
23. Question
4 pointsIdentify the correct labels of A, B and C in the following graph from the options given below:
image6 Root mean square speed (\(\begin{align}{{V}_{{rms}}}\end{align}\)); most probable speed (\(\begin{align}{{V}_{{mp}}}\end{align}\)); average speed (\(\begin{align}{{V}_{{av}}}\end{align}\)) -
Question 24 of 48
24. Question
4 pointsPoints I, II and III in the following plot respectively correspond to (\(\begin{align}{{V}_{{mp}}}\end{align}\): most probable velocity)
-
Question 25 of 48
25. Question
4 pointsInitially, the root mean square (rms) velocity of \(\begin{align}{{N}_{2}}\end{align}\) molecules at certain temperature is u. If this temperature is doubled and all the nitrogen molecules dissociate into nitrogen atoms, then the rms velocity will be:
-
Question 26 of 48
26. Question
4 pointsWhich of the following is not an assumption of the kinetic theory of gases?
-
Question 27 of 48
27. Question
4 pointsThe ratio of masses of oxygen and nitrogen in a particular gaseous mixture is 1: 4. The ratio of number of their molecules is:
-
Question 28 of 48
28. Question
4 pointsThe temperature at which oxygen molecules have the same root mean square speed as helium atoms have at 300 K is: Atomic masses: He = 4 u, O = 16 u)
-
Question 29 of 48
29. Question
4 pointsFor gaseous state, if most probable speed is denoted by C*, average speed by \(\begin{align}\bar{C}\end{align}\) and mean square speed by C, then for a large number of molecules the ratios of these speeds are:
-
Question 30 of 48
30. Question
4 pointsBy how many folds the temperature of a gas would increase when the root mean square velocity of the gas molecules in a container of fixed volume is increased from \(\begin{align}5\text{ }\times \text{ }{{10}^{4}}\text{ }cm/s\end{align}\) to \(\begin{align}10\times \text{ }{{10}^{4}}\text{ }cm/s\end{align}\)?
-
Question 31 of 48
31. Question
4 pointsWhich one of the following is the wrong assumption of kinetic theory of gases?
-
Question 32 of 48
32. Question
4 points\(\begin{align}\alpha ,v\end{align}\) and \(\begin{align}u\end{align}\) represent most probable velocity, average velocity and root mean square velocity respectively of a gas at a particular temperature. The correct order among the following is
-
Question 33 of 48
33. Question
4 pointsThe relationship among most probable velocity, average velocity and root mean square velocity is respectively
-
Question 34 of 48
34. Question
4 pointsWhen r, \(\begin{align}P\end{align}\) and \(\begin{align}M\end{align}\) represent rate of diffusion, pressure and molecular mass, respectively, then the ratio of the rates of diffusion \(\begin{align}\left( {{{r}_{A}}/{{r}_{B}}} \right)\end{align}\) of two gases \(\begin{align}A\end{align}\) and \(\begin{align}B\end{align}\), is given as
-
Question 35 of 48
35. Question
4 pointsThe molecular velocity of any gas is:
-
Question 36 of 48
36. Question
4 pointsWhich one of the following statements is NOT true about the effect of an increase in temperature on the distribution of molecular speeds in a gas?
-
Question 37 of 48
37. Question
4 pointsAs the temperature is raised from 20 ºC to 40 ºC, the average kinetic energy of neon atoms changes by which factor
-
Question 38 of 48
38. Question
4 pointsAccording to the kinetic theory of gases, in an ideal gas, between two successive collisions a gas molecule travels
-
Question 39 of 48
39. Question
4 pointsKinetic theory of gases proves
-
Question 40 of 48
40. Question
4 pointsConsider the following table:
Gas \(\begin{align}a/kPad{{m}^{6}}mo{{l}^{{-1}}}\end{align}\) \(\begin{align}b/d{{m}^{3}}mo{{l}^{{-1}}}\end{align}\) A 642.32 0.05196 B 155.21 0.04136 C 431.91 0.05196 D 155.21 0.4382 a and b are van der waals constants. The correct statement about the gases is:
-
Question 41 of 48
41. Question
4 pointsConsider the van der Waals constants, a and b, for the following gases,
Gas Ar Ne Kr Xe 1.3 0.2 5.1 4.1 \(\begin{align}b/({{10}^{{-2}}}d{{m}^{3}}mo{{l}^{{-1}}})\end{align}\) 3.2 1.7 1.0 5.0 Which gas is expected to have the highest critical temperature?
-
Question 42 of 48
42. Question
4 pointsAt a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behaviour. Their equation of state is given as \(\begin{align}P=\frac{{RT}}{{V-b}}\end{align}\) at T. Here, b is the van der Waals constant. Which gas will exhibit steepest increase in the plot of Z (compression factor) vs P?
-
Question 43 of 48
43. Question
4 pointsThe volume of gas A is twice than that of gas B. The compressibility factor of gas A thrice than that of gas B at same temperature. The pressures of the gases for equal number of moles are:
-
Question 44 of 48
44. Question
4 pointsAt very high pressures, the compressibility factor of one mole of a gas is given by:
-
Question 45 of 48
45. Question
4 pointsIf Z is a compressibility factor, van der Waals equation at low pressure can be written as:
-
Question 46 of 48
46. Question
4 pointsvan der Waals equation for a gas is stated as, \(\begin{align}P=\frac{{nRT}}{{V-nb}}-a{{\left( {\frac{n}{V}} \right)}^{2}}\end{align}\). This equation reduces to the perfect gas equation, \(\begin{align}P=\frac{{nRT}}{V}\end{align}\) when,
-
Question 47 of 48
47. Question
4 pointsThe compressibility factor for a real gas at high pressure is:
-
Question 48 of 48
48. Question
4 pointsIn van der Waals equation of state of the gas law, the constant ‘b’ is a measure of
